Chemistry Equations That Are Used in Real World Situations: A Comprehensive Overview
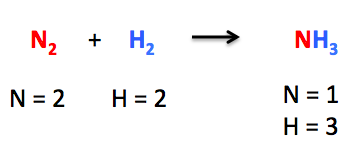
The study of the composition, structure, and characteristics of matter is a complicated and extensive area of research in chemistry. For one to understand the fundamentals of chemistry, someone must learn a number of significant chemistry equations. In various fields, including pharmacology, materials science, energy, and environmental research, a large number of chemistry equations are applied in real-world situations. Here are a few situations:
The Ideal Gas Law (PV = nRT)
In many different industries, the ideal gas law (PV = nRT) is utilized to measure and regulate the pressure, temperature, and volume of gases in numerous operations. Examples include the creation of compressed gases, the processing of natural gas, and air conditioning.
The Balancing Chemical Equations
A key idea in the synthesis and analysis of various chemicals is the Balancing Chemical Equations. This idea is used in the creation of new drugs, the manufacture of fertilizers, and the creation of diverse materials like polymers and ceramics.
The Nernst Equation
When creating batteries and fuel cells, the Nernst Equation is utilized to calculate the cell potential and enhance performance. Studies on corrosion and electroplating also make use of it.
The Arrhenius Equation
Chemical kinetics uses the Arrhenius Equation to forecast the speed of chemical reactions and to adjust the environment for those reactions in various chemical processes. For instance, it is utilized in the creation of medications, polymers, and catalysts.
Gibbs Free Energy Equation
To determine whether a chemical reaction will happen spontaneously or not, one uses the Gibbs Free Energy equation. This equation is used to comprehend the thermodynamics of numerous procedures in a variety of areas, including metallurgy, petrochemistry, and geochemistry.
Beer-Lambert law
By measuring the absorbance of light, the Beer-Lambert law is used in spectroscopy and analytical chemistry to calculate the concentration of a substance in a solution. It is used in a number of disciplines, including food chemistry, environmental science, and pharmaceuticals.
The Antoine equation
To forecast the vapor pressure of various liquids at various temperatures, the Antoine equation is utilized in the design of distillation systems. It is used in the production of petrochemicals, chemical synthesis, and crude oil refinement.
These equations serve as a foundation for developing and optimizing chemical processes, creating new materials, and comprehending the behavior of various chemicals and reactions, therefore it is important to understand and apply them in a wide range of industries and research areas.
Swikriti Dandotia



